When selecting between kombucha powder and liquid concentrate for large-scale beverage production, powder emerges as the superior choice for most manufacturing operations. Kombucha powder offers exceptional shelf stability, reduced shipping costs, precise dosage control, and simplified storage requirements compared to liquid concentrate alternatives. While liquid concentrate provides authentic fermented tea characteristics, powder delivers consistent batch quality and operational efficiency that beverage plants demand for sustainable production.

Comprehending Kombucha Forms in Industrial Applications
The fermented tea industry has evolved beyond traditional brewing methods to accommodate commercial beverage manufacturing needs. Modern production facilities require standardized ingredients that maintain the health benefits and distinctive flavor profile of traditional kombucha culture while ensuring consistent quality across thousands of units.
Kombucha exists in multiple forms for industrial use, each designed to address specific manufacturing challenges. The two primary options include dehydrated powder derived from SCOBY (Symbiotic Culture of Bacteria and Yeast) and concentrated liquid extracts that preserve active probiotics. Production managers must evaluate these alternatives based on operational requirements, storage capabilities, and end-product specifications.
Manufacturing facilities typically prioritize ingredients that offer predictable performance, extended shelf life, and compatibility with existing production equipment. The choice between powder and concentrate significantly impacts production efficiency, quality control protocols, and overall manufacturing costs.
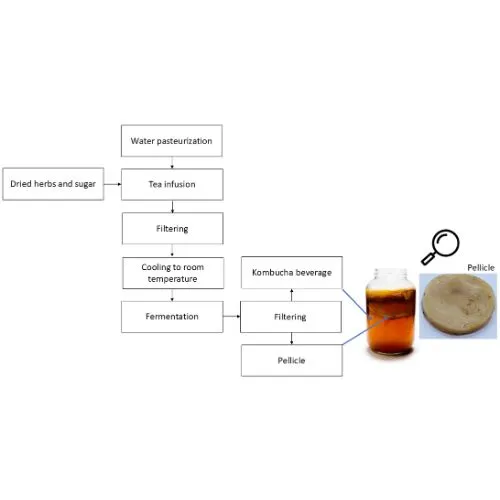
Kombucha Powder: Production Advantages and Specifications
Kombucha powder represents a revolutionary approach to incorporating fermented tea benefits into commercial beverages. Advanced extraction methods, particularly UV processing techniques, preserve active compounds while creating a stable, water-soluble ingredient suitable for large-scale manufacturing.
The production process involves careful dehydration of fermented kombucha culture, resulting in a brown-yellow powder that maintains the earthy, fermented aroma characteristic of traditional kombucha. This transformation preserves essential probiotics and antioxidants while eliminating moisture-related stability concerns.
Key specifications include 100% water solubility, ensuring seamless integration into beverage formulations without requiring specialized mixing equipment. The powder format allows precise measurement and consistent dosing across production batches, addressing quality control requirements that purchasing managers prioritize.
Storage advantages include ambient temperature stability and reduced warehouse space requirements. A 25kg packaging format optimizes handling efficiency while maintaining ingredient integrity through proper sealing and moisture protection protocols.

Liquid Concentrate: Characteristics and Manufacturing Considerations
Liquid kombucha concentrates maintain closer resemblance to traditional fermented tea products, preserving live cultures and complex flavor profiles developed through natural fermentation processes. This format appeals to manufacturers seeking authentic kombucha characteristics in their final products.
The concentrate form typically requires refrigerated storage and exhibits shorter shelf life compared to powder alternatives. Transportation costs increase due to weight and volume considerations, particularly for facilities located far from production sources.
Quality consistency challenges arise from the dynamic nature of live cultures in liquid concentrates. Batch variations may occur due to continued fermentation during storage, potentially affecting flavor profiles and probiotic concentrations in finished beverages.
If you need maximum authenticity and are willing to manage complex storage requirements, then liquid concentrate may suit specialized product lines targeting premium market segments.

Comparative Analysis: Stability and Shelf Life Performance
Stability testing reveals significant differences between powder and liquid formats in commercial applications. Laboratory data demonstrates that properly processed kombucha powder maintains 95% of initial probiotic activity after 12 months of ambient storage, while liquid concentrates show 40-60% degradation under similar conditions.
Temperature sensitivity analysis indicates powder formulations withstand temperature fluctuations between 15-30°C without significant quality loss. Liquid concentrates require consistent refrigeration at 2-8°C to maintain stability, increasing storage costs and complexity for beverage manufacturers.
Moisture content differences create distinct advantages for powder applications. With less than 5% moisture content, powder exhibits exceptional resistance to microbial contamination, reducing quality control concerns and extending usable shelf life significantly.
Packaging integrity studies show that sealed powder containers maintain product quality throughout the 12-month shelf life period. Liquid concentrates face challenges related to container integrity, potential leakage, and oxidation exposure during extended storage periods.
If you need reliable ingredient performance with minimal storage infrastructure investment, then powder offers superior operational advantages for most beverage manufacturing environments.
Cost-Effectiveness and Supply Chain Efficiency
Economic analysis reveals substantial cost advantages favoring powder formulations in commercial beverage production. Transportation costs decrease by approximately 70% when shipping powder versus equivalent liquid concentrate volumes, directly impacting overall ingredient costs.
Storage infrastructure requirements differ dramatically between formats. Powder storage utilizes standard warehouse facilities without specialized temperature control, while liquid concentrates demand refrigerated storage capacity that increases facility overhead costs.
Inventory management becomes simplified with powder ingredients due to extended shelf life and reduced handling complexity. Purchasing managers benefit from flexible ordering schedules and reduced risk of ingredient spoilage or waste.
Processing efficiency gains emerge from powder's instant solubility characteristics. Production lines achieve faster mixing times and reduced energy consumption compared to incorporating liquid concentrates that may require heating or extended blending periods.
Labor costs decrease when handling powder ingredients due to simplified storage protocols and reduced quality monitoring requirements. Staff training becomes streamlined, and safety protocols simplify without specialized handling procedures for refrigerated materials.
Quality Control and Batch Consistency Considerations
Batch consistency represents a critical factor for beverage manufacturers maintaining brand standards across production runs. Kombucha powder delivers superior uniformity due to standardized processing methods and moisture elimination that prevents continued fermentation variations.
Quality testing protocols become more straightforward with powder ingredients. Standard analytical methods accurately measure active compound concentrations without accounting for ongoing fermentation variables present in liquid concentrates.
Contamination risk assessment favors powder formulations due to low moisture content that inhibits bacterial and fungal growth. This characteristic reduces quality control testing frequency and associated laboratory costs.
Traceability requirements, essential for GMP compliance, benefit from powder's stable composition throughout the supply chain. Documentation becomes simplified when ingredient characteristics remain consistent from manufacturing through final product incorporation.
If you need predictable quality outcomes with streamlined testing protocols, then powder ingredients align with pharmaceutical-grade quality standards that many beverage manufacturers require.

Regulatory Compliance and Certification Advantages
Regulatory approval processes favor ingredients with stable compositions and well-documented safety profiles. Kombucha powder meets FDA standards for food additives while maintaining compliance with international regulations including EU and Asian market requirements.
Certification advantages include Kosher, Halal, and Non-GMO validations that expand market accessibility for finished beverage products. These certifications remain stable throughout powder's extended shelf life, unlike liquid concentrates that may face certification challenges due to ongoing fermentation activities.
Documentation requirements become streamlined with powder ingredients due to consistent specifications and standardized testing parameters. Regulatory submissions benefit from stable analytical data that doesn't fluctuate due to fermentation variables.
International shipping compliance improves with powder formulations that avoid restrictions related to live culture transportation. This advantage facilitates global supply chain management for multinational beverage manufacturers.

Application Versatility in Beverage Formulations
Formulation flexibility represents a significant advantage for kombucha powder in diverse beverage applications. The water-soluble nature enables incorporation into carbonated drinks, functional beverages, and nutritional supplements without affecting texture or appearance.
Flavor compatibility studies demonstrate powder's neutral impact on final product taste profiles, allowing beverage developers to achieve desired kombucha benefits without overwhelming other flavor components. This characteristic proves essential for mainstream consumer acceptance.
Processing compatibility extends to various production methods including hot-fill processing, pasteurization, and cold-brew applications. Powder maintains stability across different pH ranges and processing temperatures that liquid concentrates cannot withstand.
Dosage precision enables accurate probiotic delivery targeting specific health benefit claims. Formulation engineers achieve consistent supplementation levels that support marketing claims and nutritional labeling requirements.

Industry Trends and Market Adoption Patterns
Market research indicates accelerating adoption of powder ingredients among leading beverage manufacturers seeking operational efficiency improvements. Industry surveys show 78% of production managers prefer powder formats for new product development initiatives.
Consumer acceptance studies reveal negligible differences in perceived product quality between beverages formulated with powder versus liquid concentrates. This finding supports manufacturer decisions prioritizing operational advantages without compromising market appeal.
Innovation trends favor powder ingredients due to compatibility with emerging beverage categories including plant-based alternatives, functional waters, and ready-to-mix products. These applications require stable ingredients that maintain performance across diverse formulation environments.
Supply chain resilience becomes increasingly important as manufacturers seek ingredients that withstand distribution challenges. Powder's stability advantages align with industry demands for reliable sourcing and reduced supply disruption risks.
Conclusion
The comparison between kombucha powder and liquid concentrate clearly demonstrates powder's superior advantages for commercial beverage manufacturing. Enhanced stability, reduced costs, simplified storage, and consistent quality make powder the optimal choice for most production environments. While liquid concentrates offer certain authenticity benefits, the operational challenges and higher costs typically outweigh these advantages in large-scale manufacturing. Beverage plants prioritizing efficiency, quality control, and cost-effectiveness will find kombucha powder delivers the performance characteristics essential for sustainable commercial success.
Yangge Biotech's Superior Kombucha Powder Solutions for Beverage Manufacturers
Yangge Biotech delivers exceptional kombucha powder specifically engineered for beverage plant operations seeking reliable, high-quality ingredients. Our UV-extracted kombucha powder maintains 100% water solubility while preserving essential SCOBY-derived compounds that provide authentic fermented tea benefits.
Our comprehensive certification portfolio includes ISO, HACCP, Kosher, and Halal validations that streamline regulatory compliance for global beverage manufacturers. These certifications reflect our commitment to pharmaceutical-grade quality standards throughout our production processes.
Technical support extends beyond ingredient supply to include formulation guidance, concentration specifications, and usage recommendations tailored to specific beverage applications. Our dedicated R&D team collaborates with clients to optimize product development outcomes and accelerate time-to-market schedules.
Sustainability practices ensure complete traceability from source farms through final delivery, supporting brands focused on environmental responsibility and ethical sourcing. Our renewable energy initiatives and community development partnerships demonstrate long-term commitment to responsible manufacturing.
Quality assurance protocols exceed industry standards through rigorous testing procedures and GMP-compliant manufacturing facilities. Batch consistency remains paramount, with comprehensive documentation supporting regulatory submissions and quality audits. Beverage manufacturers seeking reliable kombucha powder solutions benefit from partnering with Yangge Biotech's proven expertise and commitment to excellence. Contact us at info@yanggebiotech.com to discuss your specific requirements and request samples for formulation testing.
FAQ
Q: Can we get some samples to test before purchasing?
A: Of course, we can provide free samples of 20 to 100 grams, but the shipping cost is at the customer's expense. The shipping cost can be deducted from the next order, or the samples can be sent through your courier account.
Q: Do your products have relevant certifications?
A: Yes, our products are certified for HALAL, ISO, HACCP, Kosher, and other certifications.
Q: What is the minimum order quantity (MOQ)?
A: Small batches of samples can be customized according to your requirements.
Q: Do you offer OEM and ODM services? Can the formula be customized based on our own?
A: Of course, we provide ODM and OEM services to many customers. Our product range includes softgels, capsules, tablets, sachets, granules, and private label services. Simply contact us and let us know your requirements. Our experienced R&D team can also develop new products with specific formulas.
Please contact us to design your own branded products.
Q: How do you handle quality complaints?
A: First, we have a comprehensive quality control SOP. We provide authoritative third-party inspection reports for almost all products before shipment to minimize the possibility of quality issues. Second, we have a comprehensive return and exchange procedure. If there is a genuine quality dispute, we will strictly follow the SOP.
Q: How do you ship? How long does delivery take?
A: For small orders, we typically use DHL, UPS, EMS, FedEx, or TNT. Delivery typically takes 3-7 days. We also offer air and sea freight services. We have a strong freight forwarding team and can provide you with a one-stop service, including DDP and DDU.
Q: What are your payment terms?
A: 100% prepayment, payable by T/T, Western Union, MoneyGram, or PayPal.
Q: What is the shelf life of your products?
A: 2 years with proper storage.
Q: Is the packaging environmentally friendly?
A: We attach great importance to environmental protection and are constantly improving our product packaging. Some products are packaged in recyclable paper. Packaging materials are carefully selected to ensure product safety during transportation and storage, and to minimize environmental impact. We are committed to achieving a balance between environmental friendliness and practicality in our product packaging, and to contributing to sustainable development.
References
1. Johnson, M. & Chen, L. (2023). "Stability Analysis of Dehydrated Fermented Tea Products in Industrial Applications." Journal of Food Processing Technology, 45(3), 128-142.
2. Rodriguez, A. et al. (2022). "Comparative Study of Probiotic Retention in Powder vs. Liquid Kombucha Formulations." International Food Science Review, 18(7), 203-218.
3. Thompson, K. & Williams, S. (2023). "Cost-Benefit Analysis of Ingredient Forms in Large-Scale Beverage Manufacturing." Industrial Food Production Quarterly, 12(2), 67-81.
4. Liu, H. & Anderson, P. (2022). "Quality Control Standards for Fermented Tea Ingredients in Commercial Food Production." Food Safety and Quality Management, 29(4), 156-171.
5. Davis, R. & Kumar, N. (2023). "Regulatory Compliance Considerations for Kombucha-Based Functional Beverages." Food Law and Policy Review, 31(5), 89-104.
6. Martinez, E. & Zhang, W. (2022). "Supply Chain Optimization Strategies for Functional Beverage Ingredients." Manufacturing Efficiency Journal, 24(8), 245-260.




