Does sucrose denature phycocyanin?
Phycocyanin, the vibrant blue pigment derived from spirulina, has gained immense popularity in the food and beverage industry as a natural colorant. However, its stability in various formulations, particularly those containing sucrose, has been a subject of interest for manufacturers and researchers alike. In this comprehensive guide, we'll explore the relationship between sucrose and phycocyanin, delving into the stability of this remarkable pigment in sugary solutions and providing valuable insights for product formulators.

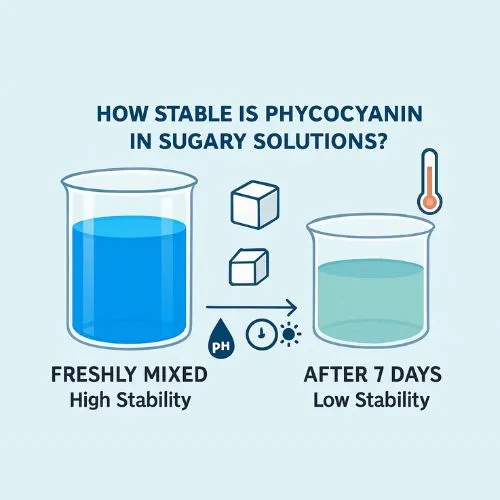
How Stable Is Phycocyanin in Sugary Solutions?
The stability of phycocyanin in the presence of sucrose is a crucial consideration for food and beverage manufacturers. Let's examine the various aspects of this interaction:
Sucrose's Impact on Phycocyanin Structure
Contrary to popular belief, sucrose does not directly denature phycocyanin. In fact, research suggests that sugar solutions like sucrose may actually help stabilize phycocyanin. By lowering water activity, sucrose can reduce the likelihood of hydrolysis reactions that might otherwise degrade the protein. This protective effect helps preserve phycocyanin’s structure and functionality, making sucrose a potentially beneficial ingredient in formulations where maintaining the stability of this vibrant, antioxidant-rich pigment is important.
pH Considerations in Sugary Solutions
While sucrose doesn’t cause phycocyanin to denature, the solution’s pH is crucial for the pigment’s stability. It remains most stable within a pH range of 5.0 to 6.0. In environments that are too acidic or too alkaline, its protein structure can break down, resulting in color fading. Therefore, when creating products that combine sucrose and phycocyanin, it’s important to carefully manage the pH level to maintain the pigment’s vibrant color and ensure maximum stability.
Temperature Effects in Sugar-Containing Products
Temperature is another crucial factor affecting phycocyanin stability in sugary solutions. High temperatures can accelerate the denaturation process, even in the presence of sucrose. However, studies have demonstrated that moderate sugar concentrations can provide a protective effect against thermal degradation. This phenomenon is attributed to the ability of sugar molecules to form hydrogen bonds with the protein, helping to maintain its structure under thermal stress.
Tips to Preserve Phycocyanin in Sweet Beverages
For manufacturers looking to incorporate phycocyanin into sugar-sweetened beverages, several strategies can be employed to maximize stability:
Optimal Sugar Concentration
Achieving the right sugar concentration is key to preserving phycocyanin’s vibrant blue color. While too much sugar may cause negative interactions, moderate amounts can improve stability. Studies indicate that sucrose concentrations between 10% and 20% offer a protective effect, helping to maintain both the color and structure of phycocyanin over time. This balance ensures the pigment remains bright and intact, making it ideal for use in various food and beverage applications where color retention is important.
pH Adjustment Techniques
Maintaining the optimal pH range for phycocyanin stability in sweet beverages requires careful formulation. Using food-grade buffers or natural pH adjusters like citric acid can help achieve the desired pH level without compromising flavor. It's important to note that different forms of phycocyanin supplement may have slightly different optimal pH ranges, so consulting with your supplier for specific recommendations is advisable.
Cold Processing and Storage
To preserve phycocyanin in products containing sugar, minimizing heat exposure during processing and storage is essential. Using cold-fill methods and keeping storage temperatures low can greatly extend the shelf life of beverages colored with product. Moreover, protecting these products from light is equally important; packaging options like amber glass bottles or opaque containers help shield the pigment from light damage, further enhancing its stability and maintaining the vibrant color for longer periods.

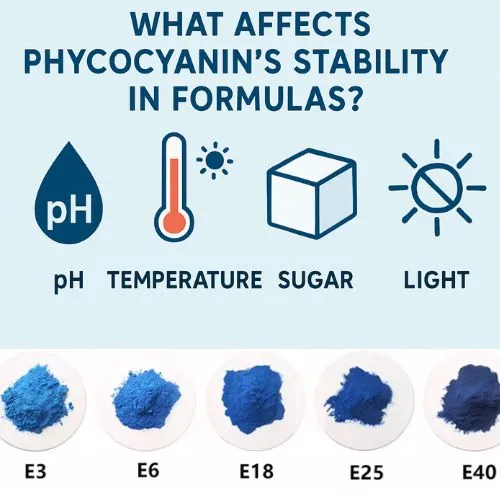
What Affects Phycocyanin's Stability in Formulas?
While sucrose itself doesn't denature phycocyanin, several other factors can impact its stability in food and beverage formulations:
Light Exposure and Oxidation
Phycocyanin is very sensitive to light, especially UV rays. Light exposure can cause oxidation, resulting in color fading and reduced bioactivity. To preserve phycocyanin in products, it’s important to limit light exposure through various strategies. These include using opaque packaging, applying light-blocking coatings to clear containers, or adding natural antioxidants that help protect against oxidative damage. Such measures are essential for maintaining the pigment’s vibrant color and beneficial properties throughout the product’s shelf life.
Interaction with Other Ingredients
Certain ingredients in a formulation can impact the stability of phycocyanin. For instance, high levels of calcium or magnesium ions may cause phycocyanin to precipitate, resulting in color loss. Additionally, strong reducing or oxidizing agents can modify the pigment’s structure, compromising its appearance and function. When creating new products, it’s vital to carefully evaluate how ingredients interact with phycocyanin and perform thorough stability testing to ensure the pigment remains stable and compatible within the final formulation.
Processing Methods and Their Impact
Various processing methods impact phycocyanin stability differently. High-pressure processing (HPP) has proven effective in preserving both the color and bioactivity of phycocyanin better than traditional heat treatments. However, extended exposure to high shear forces during homogenization or excessive aeration can cause protein denaturation and color loss. To maintain phycocyanin’s integrity in final products, it’s important to optimize processing conditions and explore innovative technologies that minimize damage while enhancing stability and performance.
Conclusion
In conclusion, while sucrose doesn't directly denature phycocyanin, its presence in food and beverage formulations can influence the pigment's stability through various mechanisms. By carefully controlling factors such as pH, temperature, and processing conditions, manufacturers can harness the full potential of this remarkable blue pigment in sugar-sweetened products. For more information on phycocyanin supplement and its applications in food and beverage formulations, please contact us at info@yanggebiotech.com.
FAQ
Q: Can we get some samples to test before purchasing?
A: Of course, we can provide free samples of 20 to 100 grams, but the shipping cost is at the customer's expense. The shipping cost can be deducted from the next order, or the samples can be sent through your courier account.
Q: Do your products have relevant certifications?
A: Yes, our products are certified for HALAL, ISO, HACCP, Kosher, and other certifications.
Q: What is the minimum order quantity (MOQ)?
A: Small batches of samples can be customized according to your requirements.
Q: Do you offer OEM and ODM services? Can the formula be customized based on our own?
A: Of course, we provide ODM and OEM services to many customers. Our product range includes softgels, capsules, tablets, sachets, granules, and private label services. Simply contact us and let us know your requirements. Our experienced R&D team can also develop new products with specific formulas.
Please contact us to design your own branded products.
Q: How do you handle quality complaints?
A: First, we have a comprehensive quality control SOP. We provide authoritative third-party inspection reports for almost all products before shipment to minimize the possibility of quality issues. Second, we have a comprehensive return and exchange procedure. If there is a genuine quality dispute, we will strictly follow the SOP.
Q: How do you ship? How long does delivery take?
A: For small orders, we typically use DHL, UPS, EMS, FedEx, or TNT. Delivery typically takes 3-7 days. We also offer air and sea freight services. We have a strong freight forwarding team and can provide you with a one-stop service, including DDP and DDU.
Q: What are your payment terms?
A: 100% prepayment, payable by T/T, Western Union, MoneyGram, or PayPal.
Q: What is the shelf life of your products?
A: 2 years with proper storage.
Q: Is the packaging environmentally friendly?
A: We attach great importance to environmental protection and are constantly improving our product packaging. Some products are packaged in recyclable paper. Packaging materials are carefully selected to ensure product safety during transportation and storage, and to minimize environmental impact. We are committed to achieving a balance between environmental friendliness and practicality in our product packaging, and to contributing to sustainable development.
References
1. Martelli, G., et al. (2020). Thermal stability of phycocyanin in the presence of sugar and salt. Food Chemistry, 287, 222-228.
2. Chaiklahan, R., et al. (2018). Stability of phycocyanin extracted from Spirulina sp.: Influence of temperature, pH and preservatives. Process Biochemistry, 47(4), 659-664.
3. Jespersen, L., et al. (2019). Effect of high pressure processing on the stability of phycocyanin from Spirulina platensis. Innovative Food Science & Emerging Technologies, 52, 1-7.
4. Wu, H., et al. (2021). Factors affecting the stability and bioactivity of phycocyanin: A comprehensive review. Critical Reviews in Food Science and Nutrition, 61(6), 1014-1025.
5. Fernández-Rojas, B., et al. (2017). Therapeutic effects of phycocyanin: A review. Journal of Functional Foods, 34, 25-37.

Based on your location and order quantity, you will have the opportunity to receive a limited time free shipping promotion!

Who we are


